Sustainability in ophthalmic optics
Sustainability in ophthalmology, what does that mean for our industry? Can we withdraw from the current debate about ecology and climate protection? Can we just ignore these topics? Undeniably, we are subject to physical-chemical laws in the manufacture and disposal of ophthalmic optical products. And supply and aftercare industries will carry on trade economically, simply out of self-interest. But do we have to tolerate unconditionally the poor working conditions in low-wage countries? Do we have any influence on business ethics there? The reputation of our industry will benefit if we discuss these environmental issues openly and look for solutions.
By Johannes Schweinem
The term ‘sustainability’ was originally coined by Hans-Carl von Carlowitz, when clearing forests to provide fuel and building materials. Around the year 1700, when neither natural gas nor oil were yet used as fuel nor concrete in the construction industry, wood was under threat because of short supply. Then coal mining took off with the advent of industrialization and the term ‘sustainability’ disappeared from view – especially as there seemed to be no limit to the supply of coal. Later, as the steam engine was supplanted by the internal combustion engine, hydrocarbons came on the scene which, as everybody knows, were – and still are – derived from oil. By 1960, there was a policy of ‘unrestrained economic growth’ and the throwaway society reached its pinnacle. The discovery of the ozone hole in 1980 and the decimation of forests in 1985 – to name just two key issues – made society aware of environmental issues. In 1990 discussions about recycling were instigated for the first time.
Cornerstones of environmental policy
In Germany, the Recycling Management Act was passed in 1996. The ‘green dot’ and the ‘yellow recycling bin’ are direct consequences of this law[1]. Research based on drilling ice cores, which took place at roughly the same time, and the insights this gave into events over the last 150,000 years, provided the final proof of anthropogenic climate change. In 1997, the Kyoto Protocol was drafted, which was subsequently adopted as the United Nations Framework Convention on Climate Change. Sustainability became an international obligation which today is integrated into the three-pillar model:
- Environmental sustainability,
- Economic sustainability,
- Societal sustainability.
Environmental sustainability calls for examination of our ‘ecological footprint’. This is the natural area that is necessary to permanently enable a person’s standard of living and lifestyle under current production conditions. This includes the production of clothing and food, the provision of energy for heating, work and leisure, as well as the disposal of pollutants (e.g. residual waste). The area of the forest required to assimilate CO2 emissions is also included in this area (Fig. 1). Some Internet platforms offer an online calculator that allows people to calculate their own ecological footprint.
Economic sustainability concerns natural resources, how long certain raw materials will continue to be available before they are exhausted (Fig. 2) and how they can best be shared, so they continue to be available to future generations. This includes, in particular, the recycling of used materials and the secondary raw materials which can be derived from them, as well as increased efficiency in the production of new goods.

Societal sustainability identifies and corrects the even distribution of the ‘footprint size’ across all nations. Ethical responsibility with regard to other cultures is just as important as the solidarity of all people with regard to public health. Europeans today want to know how our jeans are made in Bangladesh and under what conditions people in Far Eastern factories work for us. Exploitation is today largely rejected by a majority of the population. It is not always the cheapest which is best, where sustainability is concerned.
Spectacles – still the core business of ophthalmic optics
Spectacles are likely to remain the core business of ophthalmic optics for the foreseeable future. Besides good quality – perhaps the key attribute of a reputable local optometrist – the question of sustainability is becoming increasingly important. Not only do the materials used need to be biodegradable – this could be considered a shortcoming in daily use –it is also important under what conditions the lenses and frames were made. Is health protection assured for everyone involved in the production process? Are ecological and economic considerations compatible with the production process? Are the spectacles ultimately recyclable?
For an example, we should consider a pair of spectacles where the lenses are made of plastic with a standard refractive index of 1.6, the rims are made of Monel and the sides made of stainless steel. One can assume that the side tips are covered with cellulose acetate. The replacement rate for spectacles today is about four years. How, then, do the three pillars of sustainability impact on these spectacles – from manufacture to disposal?
Lenses made of plastic…
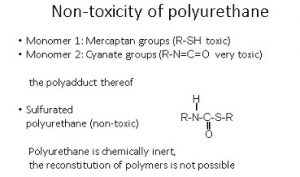
The monomers for the lenses are produced by a branch of the Japanese petrochemical industry and supplied in drums. Monomer 1 is a tetravalent molecule that reacts with terminal mercaptans. Mercaptans are short, homologous hydrocarbon chains with a terminal hydrogen sulfide grouping. The tetravalency affects the subsequent thermosetting of the finished lens. Monomer 2 is a di-isocyanate, i.e. a bivalent, reactive nitrogen-carbon-oxygen compound with a cyclic nucleus. From an ecological point of view, the chemical production of the monomers involves several intermediate stages requiring heat and releasing CO2 during the heating process. The chemical manufacturing technology in Japan as well as the occupational health and safety standards there are comparable to those in Europe.
… when casting the monomers…
In the further course of production, the monomers are supplied to those carrying out the casting in separate containers. The two monomers are poured together there into molds to make the blanks. Here, however, there are significant differences with regard to social and societal sustainability. On the one hand there are the castings manufactured under European safety standards, while on the other hand there are cheaper blanks from the Far East, manufactured without adequate health and safety protection often by underpaid employees, which are supplied to Europe.
Both the professional associations such as the Federal Institute for Occupational Safety and Health (BAuA) monitor that the workplace regulations specified in the German standards – also known as MAK values – are complied with in production. Just as any liquid can evaporate before it reaches its boiling point, i.e. takes vapor form, so too can monomers release toxic molecules into the surrounding atmosphere. The more toxic the volatile substances are, the lower the MAK values set by law. Monomer 1 is classified as “toxic”, monomer 2 as “very toxic”. The MAK value of the di-isocyanates is 0.005 ppm (parts per million) in ambient air, which may not be exceeded over an 8-hour working day. In Germany, the casting process takes place in isolated areas. In China – as a connoisseur of the Chinese casting market for optical products has reported – the two liquid monomers sometimes run directly over the employees’ hands. Here, it is right to ask whether social and societal sustainability is genuinely assured – even if business ethics are being taken increasingly seriously in China.
… and during edging
The smell of rotten eggs which arises in German opticians’ labs during edging is caused by hydrogen sulfide being given off. After all, 1.6 index plastic lenses contain 20% of sulfur, which originally stem from the mercaptans in the monomers. Although hydrogen sulfide is also toxic, the mucous membranes of our nose are so sensitive to the smell that irritation occurs long before a dangerous concentration of toxins can build up.
A study in 2004 by the Higher Professional School for Ophthalmic Optics in Cologne (HFAK) together with then professional association BGFE showed experimentally that even in the worst-case of grinding 42 high-index plastic lenses per hour in a closed room (without ventilation) only 1.0 ppm of hydrogen sulfide was emitted. The MAK value of hydrogen sulfide today is 5 ppm. So this effectively gives the all-clear for toxins in German grinding labs.
The middle part made of Monel…
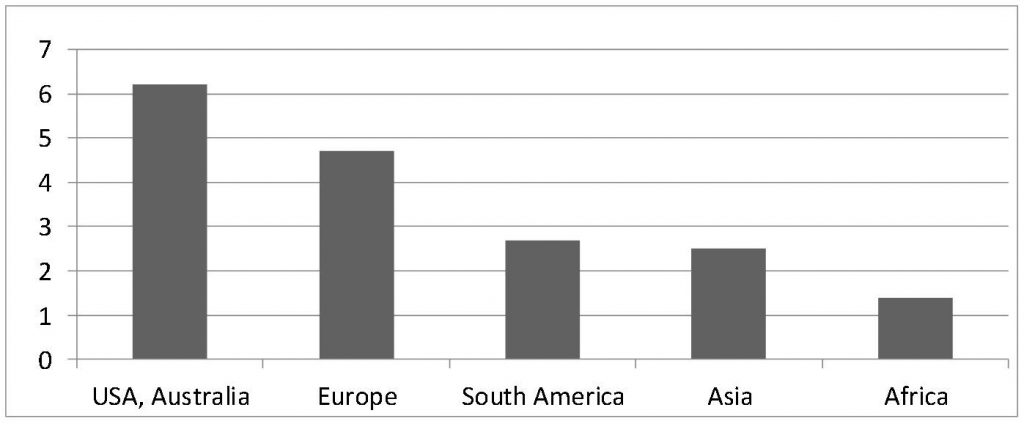
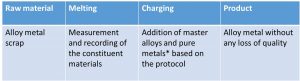
Monel has long been a common material used for the bridge of a pair of spectacles (Fig. 3). A similar alloy (nickel-silver) is used to make closing blocks, bridge and joint parts. The metals nickel, copper and zinc, mainly used in the alloys, only need be present as pure metal in small amounts. Thus the largest proportion of the nickel-based alloy Monel and the copper-based alloy nickel-silver are produced from charges of scrap metal (Fig. 4). During charging, high demands are made on the reproducibility of alloys, whereby – by presorting scrap by alloy composition and precise doses of scrap master alloys as melt addition – only a small proportion of pure metals from primary raw material sources (ores) are needed for fine adjustment. The method of scrap charging has the economic advantage that the process only needs to heat the charge to the melting point of the metal alloys, and the heat which otherwise would be needed to melt the ores – which may be four times as high – is not required. In ecological terms, too, this means that the reserves of ores can be saved in this way. In 2014, the European Copper Institute (ECI) in Brussels declared that the known reserves of copper from primary sources will have dried up in about 50 years. Consequently, the ECI is promoting the use of copper alloys from secondary sources (Fig. 5).

… and sides made of stainless steel
The same applies to spectacle sides, which in our model are made of stainless steel. The traditional coal and steel industry, i.e. the historic production of steel from the reduction of iron ore using coal in a conventional blast furnace, is no longer absolutely necessary today and has largely been replaced by electric-arc furnaces melting recycled steel scrap. The stainless steels used for spectacle frames are mainly ferritic steels made of alloy steel and chrome. Again, here too, the production of crude steel has largely been replaced by alloys.
Over the wearing life of our model spectacles – in our example four years – no di-isocyanate was given off by the glasses (Fig. 6). The finished blanks are non-toxic because the substituents of the monomers (here mercaptans) are poly-added with the isocyanates. Polyurethanes consist of the chemically stable urethane moiety within the macromolecules. Plastic lenses comply with European standards without compromise, in accordance with CE conformity and the German Medical Devices Act. Likewise, the nickel content of the spectacle frame is protected by proven coating processes.

The end of a pair of glasses…
What happens to our spectacles after four years at the end of their life? It certainly makes sense to collect old pairs of spectacles and give them to the German Opticians’ Development Service or equivalent institutions in other countries. In Germany, the Association of Opticians and Optometrists can also provide addresses of opticians who maintain contacts to Zimbabwe or Mali. Passing on technologies and goods to other countries may well be an ethical responsibility that should not be ignored.
From an ecological point of view, recycling also makes sense by ensuring that metal frames are given in large quantities to a scrap merchant, thus relieving the pressure on primary ore resources. Unfortunately, lenses made from plastic cannot be recycled because as thermosets they cannot be remelted. So what happens to them? And how do customers finally dispose of their old glasses? The legislation does not require the individual parts of the spectacles to be separated and often they simply end up in the residual waste. So let us now take a closer look at this final stage in the life of a pair of spectacles.
… into the incinerator
The content of a residual waste bin is transported by truck to a collecting area, from where it is taken by train to a large waste incineration plant. The Recycling Management Act of 1996 requires sorting of residual waste for recyclable materials; residual waste may no longer be dumped untreated into landfills. Waste incineration plants are now well established, ultimately leading to a reduction in the overall amount of waste. At these waste-disposal plants, the incoming waste is initially sorted on shakers using magnets (Fig. 7). As likely as not, the ferritic of the sides will be attracted to the magnets and thus the whole spectacle frame will be fished out. However, if the spectacles were first crushed in the trash – with the steel sides becoming separated – the central part of the spectacles may end up in the incinerator.

On rotating grids, the burning garbage is constantly turned over. The plastic parts of the frame (side tips, nose pads and plastic lenses) burn mainly to carbon dioxide and water vapor. The cellulose acetate side tips account for about 45% by weight of the carbon-neutral CO2 which the cotton plants absorbed during growth (Fig. 8). More problematic are the lenses whose cyclic components of the plastic matrix in combination with chlorine from the overall waste can produce dioxins and furans.
The legislation specifies strict regulations concerning the retention of pollutants in waste incineration plants. Thus, the incineration chambers are designed in such a way that temperatures of up to 1200 °C occur above the flames, to ensure thermal decomposition of any dioxins or furans. The high temperatures, however, also have the side effect that nitrogen oxides are produced. Thus sulfur dioxide, sulfide residues and suspended particles are also left behind by our 1.6-index lenses. For this reason, filters are installed in waste incineration plants, such as suspended-particle scrubbers, hydrogen halide and sulfur dioxide separators, resin filters for heavy metal removal, ammonia catalytic crackers for the removal of nitrogen oxides and active carbon filters through which all gases have to pass before water vapor and carbon dioxide leaves the chimney. A little digression concerning plastic frames: it can be calculated stoichiometrically that a 12 g polyamide spectacle frame gives off 28 g CO2 during combustion.
Thermal recovery
From an economic point of view, when plastics are burnt, on average 60 % of the process heat required to produce the plastic is recovered as a combination of heat or power, and then returned to the grid as electrical energy. The remaining metal components of our model spectacles can also be recovered from the ashes and slag. They, too, can thus be returned to the metallurgists for use as a secondary raw material.
Polyethylene and polypropylene (plastic bags) burn with few emissions to H2O and CO2, whereby their calorific value is equivalent to heating oil (around 40,000 kJ/kg). If necessary, dirty packaging from the yellow recycling bin can be added, if the delivered residual waste mixture proves inefficient as a fuel.
Conclusion
Sustainability has now become a high priority. Today’s industrial practices are setting new economic-ecological standards. The questioning of the ethical production of cheap plastic lenses in part is up to us. We are all challenged to confront the key issues of the 21st century, to ensure that life for future generations remains worth living.
[1] All references to government or legislation in this article refer to Germany unless stated otherwise.



