The Craftsmanship of Ophthalmic Coatings
Principal knowledge on procedures and best practise
The objectives of this article are to provide general principal knowledge on ophthalmic coating manufacture procedures and best practises based on a hands-on lifetime experience in coating manufacturing. Moreover, it is meant to draw attention to pitfalls and possible risks, to show shop-floor level staff how to apply themselves, to take ownership of the work and to enable suitable candidates to be an efficient coach in the lab. At the end of the day the quality level achieved in a coating department is determined by the quality of workmanship of the least trained staff. By Georg Mayer
The text is an extract of a tutorial held first at the Annual Society of Vacuum Coaters (SVC) Techcon 2019 in Long Beach and to be presented again in an updated version at this year’s SVC Techcon April 22nd in Chicago. I will focus on the next few pages on some highlights of the full day course tutorial. In nearly 20 years of sole responsibility of the coating division of a then market leader, I gained some valuable lessons. Having had the privilege and pleasure to work with staff from 5 European and African Countries was an enriching and exciting experience.
Some ground rules for every case
Here are a few conclusions after working nearly 40 years in coating
- Be a professional pessimist, expect the worst to happen and plan accordingly.
- Don´t assume, ask! There are no stupid questions, only stupid mistakes.
- Be a stickler to the rules of the process owner, don´t change anything, it is always better to ask again.
- Consistency is the name of the game, more of that later …
You might ask yourself how can this experience still be of any interest today, in our increasingly automated Rx factories, gearing up for Industry 4.0?
Rx lens making is mostly automated – a computerised digital production with the ability to check and verify every lens to all standards applicable in situ. Rx surfacing runs in a continuous flow, job by job and can be driven by only a few staff with the help of advanced fully integrated lab management software.
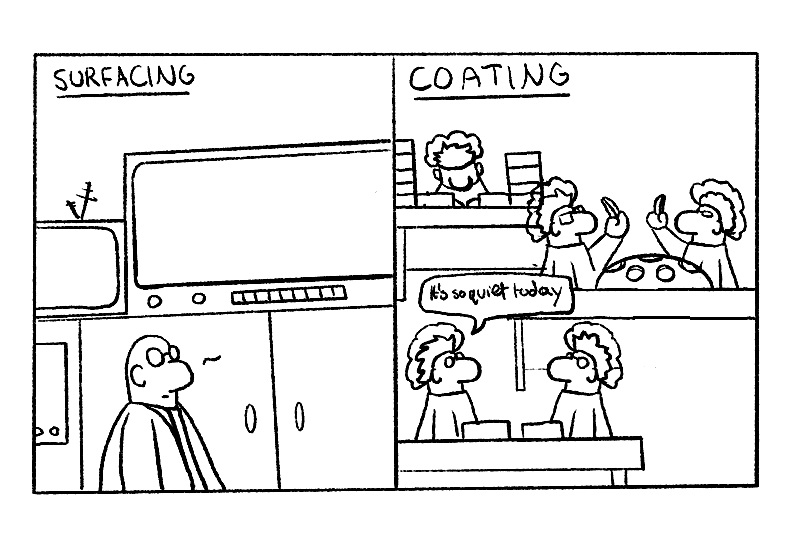
However, when the Rx jobs arrive at the coating department´s door we seem to go back in time.
We interrupt the job flow for batching, not only once but often twice with related waiting times and sorting action per material/index and per coating type. Due to the unique shape and form of Rx lenses we still have too many hands on those lenses, all the way through the coating department. First they go from batching into carriers to cleaning to hardcoating and curing. And then again they go from batching and handling into different carriers for preparation and vacuum coating, typically twice with manual flipping and prep-steps in between.
In summary, we have multiple manual manipulations on lenses and machines, meaning a lot of staff are in direct control of the coating quality. And if those manual manipulations on lenses, machines or processes are performed in a country by operators with language barriers, training needs particular attention as those staff form a crucial part of a consistently good quality.
On top of all of this we don´t have the ability to check the full and final quality of each of our coatings produced without destroying it, we can only test samples or “witness” substrates which have taken part in the same process and batch.
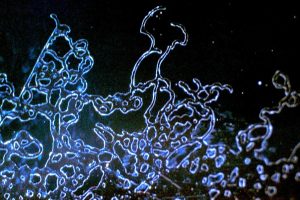
Test yourself: what do you think is depicted in these pictures? You can find the solutions at the end of this article.



Keep an eye on sampling method, process and FPY
This leads directly to a first major subject, – the sampling method as a basis for coating quality metrics.
- Sampling only works if all lenses are processed consistently to the rules of the process owner
- Only then such witness samples will have the same properties as all other “real” Rx lenses
In conclusion, if processes and staff are not consistent the sampling approach is misleading.
A second important subject is the previously mentioned process and the lab taking ownership and responsibility. The following list gives an overview of the most important points that should be considered regarding the process:
- Apply stable robust processes, manageable by the local infrastructure, machines and team.
- The process must be well documented and the documentation has to be easily understandable and available to staff at their workplace.
- Part and parcel of a stable process is strict maintenance of machines and use of the correct consumables since you can´t separate machine from process.
- Well trained staff is essential, each of them could be the weak link in an otherwise strong team.
- Create a work atmosphere that is open to continuous questions, learning and improvement, all the time.
Such stable and robust processes are the basis for a third most critical topic, the first pass yield (FPY) and its primary impact on an Rx lab’s ability to deliver consistently on time which leads to customer satisfaction.
A simplified example/case for this is based on the Markov Chain:
Of 100 lenses to enter a coating department, how many will come out “first round / first try” with its typical successive process steps? Let´s assume each main coating production steps FPY being
Hard coat 96%
First side AR 99%
Second side AR 99%
Handling/washing 99%.
Looks pretty good, doesn´t it, but the key is that in combination the final score is a multiplication of 0,95×0,99×0,99×0,99=0,92 or 92%, meaning 8 of our 100 lenses didn´t make it first time round.
To make matters worse, in Rx where jobs are 2 lenses it could be double the number as a single bad lens in a job will hold back the other good lens too. So, this coating department could have a FPY as low as 84%, or in other words 16% of all work is not out first round in the expected time.
Once the whole Rx lab’s various factors have been modelled the results can be used for the prediction of the customer’s perception of this lab, i.e. if customers’ expectations will be met.
The main delivery quality indicators are delivery speed, reliability and consistency.
Such a lab might be competitive fast with the jobs making it on first attempt (84% FPY), but with a significant 16% of work not on time the lab will be seen as unreliable, and worse, of those 16% another 2% will not make it through even the second time and will need a third round. And because of this noticeable tail of very late work the lab is also regarded as not consistent.
Conclusion and brief outlook
Rx labs coating management must focus all efforts to achieve and maintain the highest possible yield rate for each type of product made by the lab, based on stable processes and well-trained staff, because of its primary impact on delivery quality and customer satisfaction.
Once this goal is achieved the focus can shift to removing the remaining major system shortfalls in most coating departments, i.e. to the improvement of the degree of automation and the reduction of the process times while still maintaining expected market quality standards for Rx lenses.
We can foresee some exciting new developments and projects in that direction, partly already in use or still to come, which will change the landscape of Rx lens coating to bring it closer to the level of automation seen already in Rx surfacing.
Solution for pictures a) to d):
a) Hardcoating failure
b) AR stack thermal cracking
c) Hardcoating striae
d) Droplet spitmark residue under AR