Overcoming material barriers in myopia control lenses

Asymmetric Peripheral Defocus Lenses (MPDLs), an emerging alternative in myopia control
The last decade has seen a surge in innovations designed to slow the progression of myopia. A broad spectrum of ophthalmic solutions – including defocus-incorporated multiple segments, concentric ring designs, and various progressive-like structures – has emerged, each offering a distinct approach to inducing peripheral myopic defocus. These designs are supported by evidence indicating that optical strategies can reduce axial elongation, which is closely associated with myopia progression.
Despite this progress, the industrial implementation of many of these lens concepts remains limited. Most are manufactured from special semi-finished blanks with fixed geometries. This production method not only restricts the range of available materials (for example, different indices, photochromic, or polarized variants) but also limits accessibility for laboratories, as such lenses typically require semi-finished blanks that are not widely available. Consequently, producing and distributing clinically validated lens designs can be challenging, particularly for laboratories equipped only with standard free-form production capabilities.
IOT’s Asymmetric Myopic Peripheral Defocus Lenses (MPDLs) offer a practical solution to these barriers. Unlike designs requiring semi-finished blanks, MPDLs are manufactured entirely using free-form surfacing technology. This development has considerable implications: it enables laboratories to produce lenses for myopia control across a wide variety of materials already integrated into their production lines, eliminates reliance on special semi-finished blanks, and increases accessibility of the technology across the optical industry.
Lens design and manufacturing concept
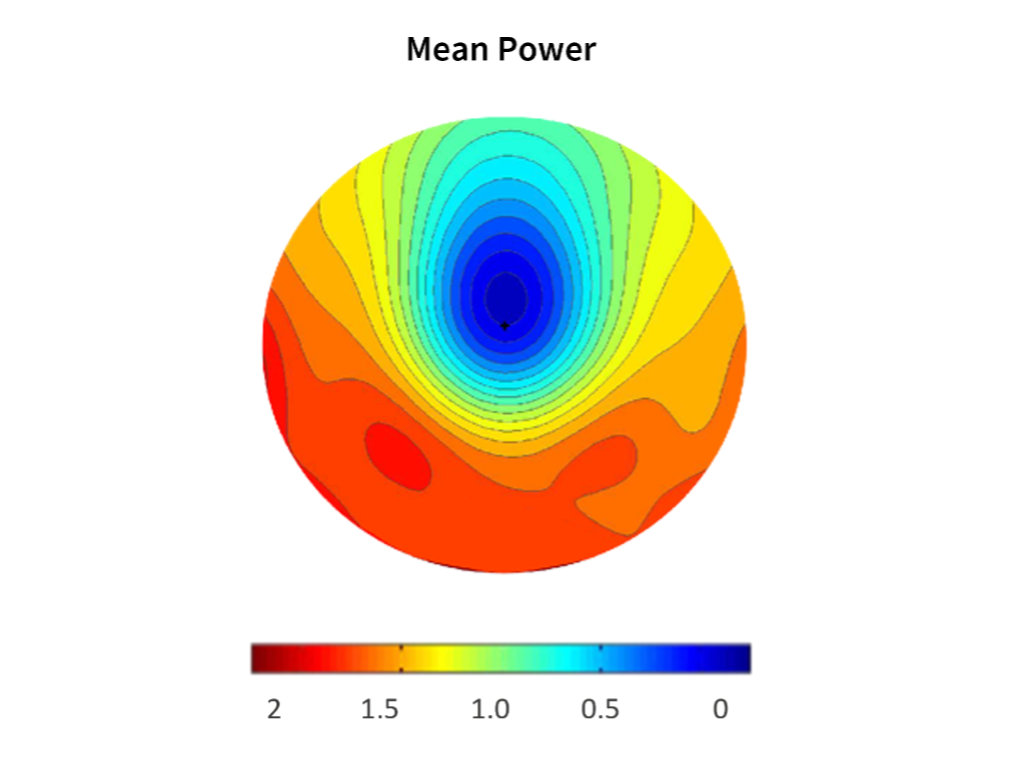
The foundation of MPDLs is a carefully engineered optical geometry that induces asymmetric myopic peripheral defocus. The central 7 mm optical zone is reserved for distance vision correction, while the surrounding zones introduce positive defocus values intended to slow axial elongation. Specifically, the design incorporates +1.50 D in the nasal periphery, +1.80 D temporally, and +2.00 D inferiorly.
This asymmetric configuration considers variations in eye geometry and distributes peripheral defocus across multiple zones. By varying the positive power across meridians, MPDLs provide a defocus profile designed to balance optical efficacy and visual performance. With the central zone free of defocus, distance correction is preserved, while peripheral zones generate the myopic defocus profile.
From an industrial perspective, the main strength of this design lies in its implementation through free-form technology. Free-form surfacing enables laboratories to machine complex geometries directly onto spherical semi-finished blanks, regardless of their base material. The same process routinely used for progressive addition lenses or advanced single-vision designs can be applied to MPDLs.
The result is a lens design that:
- Can be manufactured in multiple refractive indices, ranging from standard 1.50 to high-index 1.67 or 1.74.
- Can be integrated with photochromic or polarized treatments, accommodating outdoor usability and lifestyle requirements.
- Remains accessible to independent laboratories, which can incorporate MPDLs into their portfolio without new molds, special semi-finished stock, or dedicated equipment.
- This versatility makes MPDLs not only a clinically relevant innovation but also an industrial solution ready for widespread adoption within the ophthalmic industry.
Clinical validation in a European population
Clinical validation is a prerequisite for any myopia control lens. While many optical designs have demonstrated success in Asian populations, relatively few randomized controlled trials have been conducted in European children. This is a crucial gap, as ethnic, environmental, and behavioral factors may influence both myopia progression rates and treatment efficacy.
IOT’s MPDLs were evaluated in a randomized, double-masked clinical trial conducted in Spain. The study enrolled 92 children aged 6 to 12 years with progressing myopia (after 24 months of follow-up, 69 children remained in this study), randomly assigning them to wear either MPDLs or standard single-vision lenses. Subjects were followed for a total of 24 months, with periodic evaluations of refractive status, axial length, and visual performance.
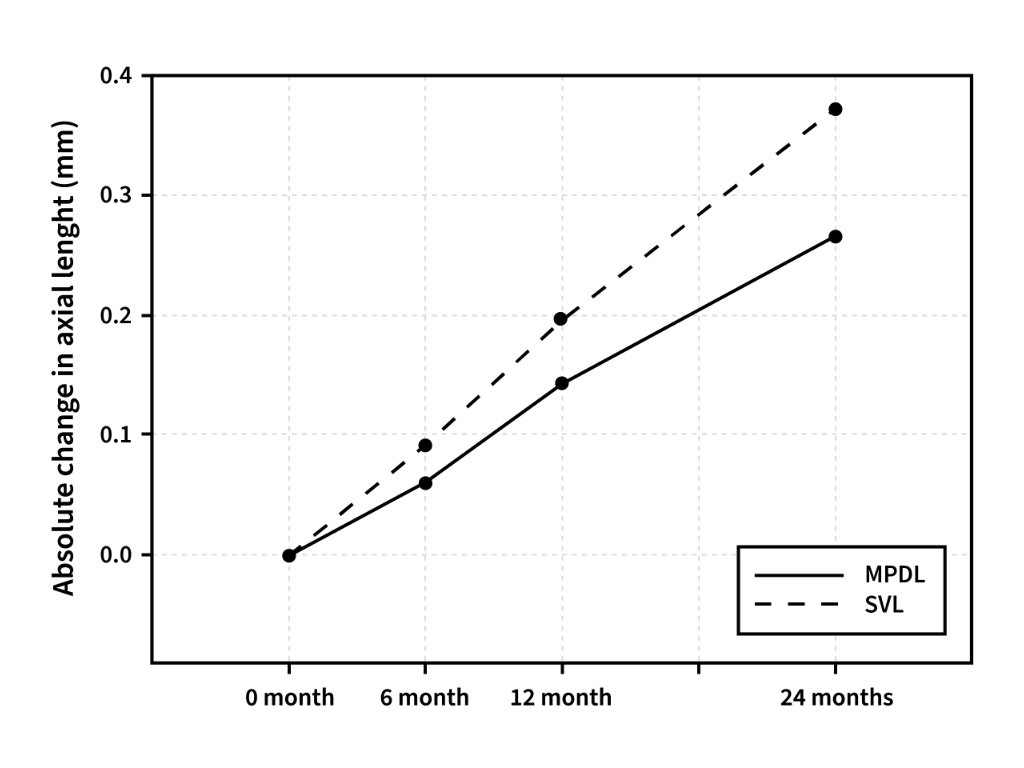
Initial outcomes after 12 months showed a significant reduction in axial elongation among children wearing MPDLs compared with controls. Importantly, the recently reported 24-month results confirm that the beneficial effect is not transient but sustained over time. This makes MPDLs one of the few lens designs with long-term clinical validation in a European cohort.
Key clinical outcomes
The primary endpoint of the study was axial length growth, considered the most robust biomarker for myopia progression.
At 12 months, children wearing MPDLs demonstrated a 39% reduction in axial elongation compared with the control group fitted with single-vision lenses.
At 24 months, the efficacy remained substantial, with a 29% reduction in axial elongation, indicating that the treatment effect is maintained over a two-year period.
These results confirm that MPDLs provide a consistent optical intervention capable of slowing axial growth in children over an extended period, supporting their use as a clinically validated myopia control lens.
The study also highlighted the role of age at initiation. Younger participants exhibited stronger treatment responses, indicating that early adoption of MPDLs may maximize long-term benefits. This finding supports the notion that timely intervention is critical for controlling axial growth trajectories in children.
While axial length was the primary focus, refractive error outcomes mirrored the elongation data, with MPDL wearers showing less myopic shift than controls. Importantly, visual acuity remained uncompromised, and subjective acceptance of the lenses was high, underscoring their practical viability for myopia control in children.
Implications for the optical industry
The implications of these findings extend beyond the clinic and into the manufacturing domain. MPDLs represent a unique convergence of scientific validation and industrial feasibility.For the ophthalmic lens industry, MPDLs address a persistent challenge: delivering effective myopia control solutions without being limited by material constraints. By utilizing a free-form-only design, laboratories can:
Offer a broad portfolio: MPDLs can be produced in standard, mid- and high-index materials, as well as specialty variants such as photochromic or polarized lenses. Integrate with existing workflows: no special semi-finished blanks or molds are required, reducing costs and simplifying production. Enhance accessibility: the design can be manufactured by any laboratory equipped with free-form technology, supporting broader availability of advanced myopia control lenses.
This flexibility supports the integration of MPDLs across laboratories equipped with free-form technology, enabling production using existing equipment and established workflows.
Conclusion
Asymmetric Myopic Peripheral Defocus Lenses represent a breakthrough that extends beyond optical design. Clinically, they have demonstrated sustained efficacy in slowing axial elongation over 24 months in a European myopic population. From an industrial perspective, their design overcomes the limitations of special semi-finished blanks, allowing free-form manufacturing across a wide range of lens materials.
By uniting these two dimensions – clinical validation and industrial feasibility – MPDLs position themselves as a highly practical solution for myopia control. They offer laboratories the ability to meet growing demand with a versatile, accessible design while ensuring that children benefit from a treatment that is both effective and comfortable.
In an era where myopia prevalence continues to rise and industry seeks scalable solutions, MPDLs demonstrate that overcoming material barriers is not only possible but also essential for the next phase of myopia management.
References: Sánchez-Tena, MA., Cleva, JM., Villa-Collar, C., Álvarez, M., Ruiz-Pomeda, A., Martinez-Perez, C., Andreu-Vazquez, C., Chamorro, E., & Alvarez-Peregrina, C. (2024). Effectiveness of a Spectacle Lens with a Specific Asymmetric Myopic Peripheral Defocus: 12-Month Results in a Spanish Population. Children, 11(2), 177. Martinez-Perez, C., Sánchez-Tena, MA., Cleva, JM., Villa-Collar, C., Álvarez, M., Chamorro, E., & Alvarez-Peregrina, C. (2025). Efficacy of Asymmetric Myopic Peripheral Defocus Lenses in Spanish Children: 24-Month Randomized Clinical Trial Results. Children, 12(2), 191. Concepción-Grande, P., Cano, C., Sánchez-Tena, M., Álvarez-Peregrina, C., Andreu-Vazquez, C., Cleva, J., & Villa-Collar, C. (2024). Subjective wearing experience of a novel spectacle lens for myopia management based on peripheral asymmetric myopic defocus. European Academy of Optometry and Optics (EAOO).